GMP 标准
GMP 代表良好生产规范,是一种确保制造的产品(例如食品、化妆品和药品)始终按照既定的质量标准进行生产和控制的系统。实施 GMP 有助于减少损失和浪费,避免召回、罚款和入狱。总的来说,它可以保护公司和消费者免受负面食品安全事件的影响。
GMP 检查并涵盖制造过程的每个方面,以防止任何可能对产品造成灾难性影响的风险,例如交叉污染、掺假和贴错标签。
|
| |
GMP A (ISO 5 )In theory, for a classified room (not just below a LAFW hood) to reach ISO class 5 air cleanliness, you need to enter the cleanroom via an ISO 8 (ante-room), then go through an ISO 7, followed by an ISO 6 to finally get into the ISO class 5 cleanroom. In reality, however, you can reach an ISO 5 cleanroom with 2 or 3 airlocks. The optimal layout depends on the process taking place inside the cleanroom, the size of the room, the number of people working inside, the equipment inside, etc. The filtered air sweeps down the room in a unidirectional way, at a velocity generally between 0.3 m/s and 0.5 m/s, and exits through the floor, removing the airborne contamination from the room. Cleanrooms using unidirectional air flow are more expensive than non-unidirectional ones, but can comply with more stringent classifications, such as ISO 5 or lower.
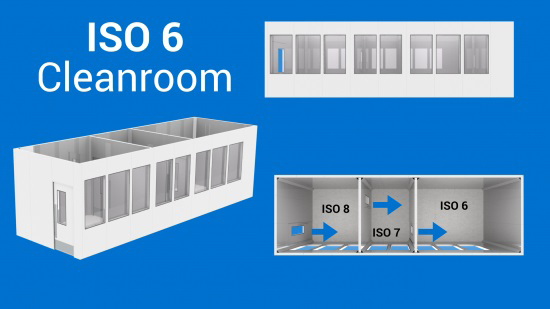
Some of our ISO 5 Cleanroom past projects : ISO 5 Nanofabrication cleanroom facility | GMP B (ISO 6 )In theory, for an entire room to reach ISO 6 air cleanliness, you need to enter the cleanroom via an ISO 8 (ante-room), then go through an ISO 7, to finally get into the ISO 6, as shown in the image. In reality however, you can reach an ISO 6 cleanroom with 1 (recommendation is 2) airlock. Again, it depends of the size of the room, the process taking place inside the cleanroom, the number of people working inside, the equipment inside, etc. Unidirectional air flow is sometimes recommended to reach ISO 6 classification. For a room of less than 4–6 meters in width (depending on the activities taking place inside the cleanroom), air returns can be positioned on the side of the walls instead of in the floor. Installing air returns in the floor is more expensive.
Some of our past ISO 6 cleanroom projects: Biopharmaceutical cleanrooms Animal laboratories ISO 6 cell production cleanrooms | |
|
| |
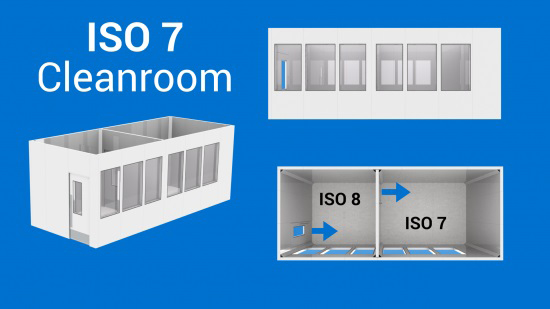
GMP C (ISO 7 )This is one of the most common classes of cleanrooms. If you need an ISO 7 cleanroom, you should consider having an ISO 8 airlock/gowning room prior to entering the ISO 7 room. The air changes per hour will vary in both rooms as described below.
Non-unidirectional air flow Some of our ISO 7 Cleanroom past projects : Clinical Manufacturing Facility for Cell and Gene Therapy – CGMP cleanroom ISO 7 cleanroom for Nutraceutical Industry Clinical Trials – Drug Development cleanroom ISO 7 sterile compounding non-hazardous facility In-Hospital compounding cleanroom – ISO 7 ( USP-797 ) | GMP D (ISO 8 )Let’s assume that an unclassified space (office or lab) is ISO 9. In this case, you can directly enter an ISO 8 cleanroom, without an airlock. Depending on the production process inside the cleanroom, however, you may have to add a gowning room.
Non-unidirectional air flow Some of our ISO 8 Cleanroom past projects : ISO 8 cleanroom for Pharmaceutical Manufacturing Cleanroom for Pharmaceutical Industry Cleanroom wall addition for Medtech Manufacturer ISO 8 cleanroom for Medical Device Manufacturing |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
FAQ
食品和药品的 GMP 标准规范了哪些流程?
质量管理、卫生和卫生、建筑和设施、设备、原材料、人员、验证和资格、投诉、文档和记录保存、检查和质量审核。
GMP 和 CGMP 有什么区别?
在大多数情况下,良好生产规范 (GMP) 和现行良好生产规范 (cGMP) 可以互换。GMP 是指美国食品和药物管理局 (FDA) 根据《联邦食品、药品和化妆品法》颁布的基本法规,旨在确保制造商采取积极措施保证其产品安全有效。另一方面,cGMP 由 FDA 实施,以确保制造商不断改进产品质量方法。它意味着通过使用最新的系统和技术,不断致力于达到最高的质量标准。
良好生产规范的 5 个主要组成部分是什么?
2对于制造业来说,在工作场所规范 GMP 以确保产品始终如一的质量和安全至关重要。GMP 的五个主要组成部分(通常称为 5P)可帮助组织在整个生产过程中遵守严格的标准。
什么是 GMP 标准?
GMP 标准的制定是为了提高制造产品(尤其是药品)的安全性,并确保消费者获得尽可能高的质量。遵守 GMP 标准不仅会对制造公司的声誉产生积极影响,还可以减少批次召回和消费者的负面报告。以下是您可以遵循的 4 项措施来维护 GMP 标准
如何进行 GMP 认证?
验证是展示经常使用或执行的仪器、流程和活动的记录行为。这样做是为了检查它们是否按预期运行。 GMP 可能涉及许多需要验证的内容,但最好重点关注以下流程:
流程验证
清洁和卫生验证
计算机系统验证
分析方法验证
如何遵守指南?
GMP 指南和法规解决了可能影响产品安全性和质量的不同问题。符合 GMP 或 cGMP 标准有助于组织遵守立法命令、提高产品质量、提高客户满意度、增加销售额并获得丰厚的投资回报。
Related Information
-
共0页 0条
 18186671616
18186671616 pengwu1616@gmai.com
pengwu1616@gmai.com
 MENU
MENU